Case Report and Discussion
Hypertrophic cardiomyopathy (HCM), a genetic disease caused by mutations in the genes encoding cardiac sarcomere proteins, is characterized by myocardial hypertrophy and intermittent ventricular outflow obstruction. It is the leading cause of sudden cardiac death (SCD) in young individuals with an estimated global prevalence of 1 in 500 individuals. Molecular testing such as next-generation sequencing (NGS) plays a crucial role in the diagnosis and management of HCM in clinical settings [
1]. In forensic practice, postmortem NGS can identify potential pathogenic mutations that may contribute to sudden death and provide valuable insights into the cause of death and potential risks to surviving family members.
This report documents the case of a 40-year-old male with no significant past medical history who suddenly fell out of bed while asleep at home and was found unresponsive by his wife. He was transferred to a hospital and pronounced dead on arrival. The deceased had a family history of HCM and sudden death; his eldest brother had had a confirmed diagnosis of HCM, and the second brother had experienced sudden death.
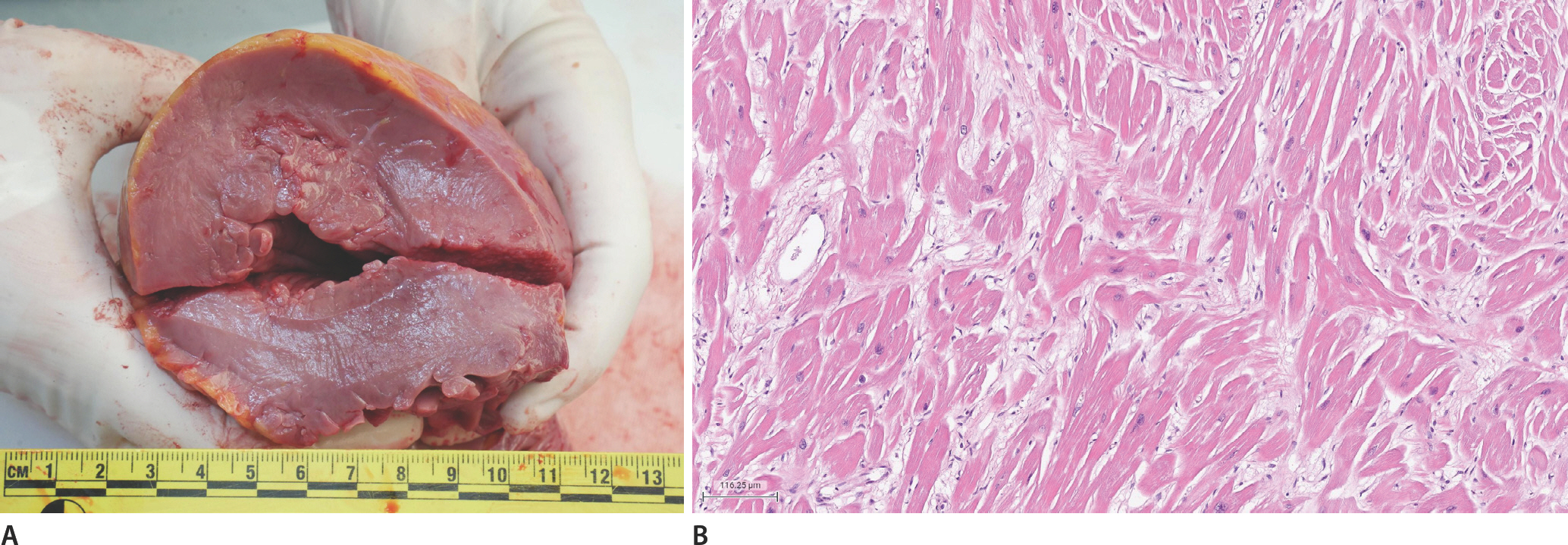
An autopsy was conducted one day after death. The deceased weighed 92 kg and was 181 cm tall. External examination findings were unremarkable, except for injuries consistent with cardiopulmonary resuscitation. The heart weighed 732 g and showed asymmetric septal hypertrophy without significant coronary artery atherosclerosis. The thicknesses of the left and right ventricular walls and the septum were 1.8 cm, 0.5 cm, and 2.7 cm, respectively (
Fig. 1A). Hematoxylin and eosin-stained sections of the myocardium showed cardiac myocyte hypertrophy, myocyte bundle disarray, and interstitial fibrosis (
Fig. 1B). The rest of the internal organs and the results of the postmortem ancillary tests were unremarkable.
Fig.┬Ā1.
Gross examination of the heart shows asymmetric septal hypertrophy with left ventricular wall and septal thickness of 1.8 cm and 2.7 cm, consistent with hypertrophic myopathy (A). Cardiac myocyte hypertrophy, myocyte bundle disarray, and interstitial fibrosis are noted in the left ventricular wall (B; H&E, ├Ś100).
During autopsy, cardiac blood was collected and sent to a commercial laboratory for postmortem NGS. This comprehensive genetic testing identified a pathogenic mutation, c.2833_2834del, in the
MYBPC3 gene, which encodes myosin-binding protein C3. The presence of this mutation confirms the diagnosis of HCM and provides an explanation for the sudden death. The identified mutation, c.2833_2834del, caused a shift in the reading frame starting at codon arginine 945, changing it to glycine, resulting in a premature stop codon at position 105 in the new frame (p.Arg945GlyfsTer105). This variant is expected to cause a loss or disruption of the normal protein function through nonsense-mediated decay or protein truncation. This loss-of-function variant is classified as a pathogenic variant in ClinVar (ID: 42660) and is associated with HCM type 4 according to Online Mendelian Inheritance in Man (OMIM). Although mutations in the
MYBPC3 gene account for 30% to 40% of HCM cases, the identified c.2833_2834del mutation is rare, with a minor allele frequency of 0.0004% in the general population and no previous reports in the Korean population [
2]. The inheritance pattern of this mutation is autosomal dominant, indicating that it can be inherited within the family. Considering the familial implication, genetic counseling for the bereaved family members is strongly recommended, particularly for the nine-year-old son of the deceased, who has a 50% chance of inheriting the mutation.
The identification of the novel pathogenic mutation in the MYBPC3 gene, not previously reported in the Korean population, underscores the significance of our case report in expanding the knowledge base of HCM genetics within this specific population. This novel mutation contributes to the existing understanding of the genetic heterogeneity of HCM and highlights the importance of considering population-specific variations in genetic screening and diagnostic approaches. By reporting this novel mutation, we contribute to the growing body of evidence regarding the diverse genetic landscape of HCM and emphasize the need for further studies to elucidate the prevalence, penetrance, and clinical outcomes of this specific mutation in Koreans.
NGS is an advanced and rapidly evolving technology that facilitates high-throughput and cost-effective genetic analysis. The introduction of postmortem NGS has revolutionized the field of molecular autopsy as it enables the simultaneous analysis of multiple genes associated with various genetic disorders, such as cardiomyopathy, channelopathy, and other cardiovascular diseases. This groundbreaking approach has the potential to significantly enhance the accuracy of causes of death by identifying previously unknown genetic factors. Moreover, postmortem NGS contributes to the expansion of our understanding of genetic diseases and has implications for improving public health prevention strategies. Additionally, by uncovering genetic variants and their implications, postmortem NGS may facilitate access of affected family members to tailored and informed genetic counseling, thereby empowering them to make informed decisions regarding their health and potential risk factors. The emergence of postmortem NGS represents a remarkable advancement in the field of genetic analysis, offering immense potential in forensic medicine and paving the way for more comprehensive investigations into the genetic underpinnings of sudden and unexplained deaths.
Numerous clinical guidelines endorse the utilization of molecular autopsy for suspected cases of SCD. In 2015, the Swiss Society of Forensic Medicine recommended autopsy for all sudden death patients aged <40 years and emphasized proper sample collection and storage for molecular testing, effective communication with families, and a multidisciplinary approach with genetic counseling [
3]. In the same year, the European Society of Cardiology guidelines proposed a targeted genetic analysis for specific inheritable channelopathies or cardiomyopathies in all sudden death cases [
4]. In 2017, the American College of Cardiology/American Heart Association/Heart Rhythm Society guidelines recommended postmortem genetic testing for SCD patients with heritable cardiomyopathies or absence of structural disease, indicating potential cardiac channelopathy [
5]. Two years later, European recommendations emphasized full autopsies, sample storage, and multidisciplinary investigations of SCD cases [
6]. In 2022, an expert consensus statement on the state of genetic testing for cardiac diseases advised the retention of ethylenediaminetetraacetic acid blood and/or fresh tissue for genetic analysis, targeted testing for likely genetic causes, and extended panel screening for unexplained SCDs in patients aged <50 years [
7]. Additionally, the Royal College of PathologistsŌĆÖ guidelines on autopsy practice recommend the preservation of fresh blood and/or splenic tissue for molecular autopsy in individuals aged <40 years with suspected inheritable heart disorders or thoracic aortic dissection [
8].
Current international guidelines emphasize the crucial need of conducting comprehensive autopsy and considering postmortem genetic testing in cases of SCD with suspected inherited cardiac disorders, especially in cases where autopsy does not reveal structural abnormalities or when specific inheritable cardiomyopathies or channelopathies are suspected. Furthermore, these guidelines emphasize the importance of proper sample collection and storage for future genetic analysis as well as adoption of a multidisciplinary approach involving genetic counseling and collaboration with the cardiogenic team. Collectively, these guidelines underscore the significant role of integrating molecular autopsy and genetic testing in the investigation of SCD, as they enhance diagnostic accuracy, provide insights into the underlying genetic factors, and enable appropriate genetic counseling for affected family members. Following these guidelines, forensic medicine and clinical practice can effectively leverage the potential of postmortem genetic analysis to elucidate the genetic basis of SCD and improve patient care.
This case report is the first autopsy study in Korea to incorporate postmortem NGS in an SCD case, marking a significant milestone in forensic practice. The identification of a novel pathogenic mutation in the MYBPC3 gene that would have remained undetected using conventional methods alone underscores the critical importance of postmortem NGS and highlights the necessity for its incorporation into forensic practice. By integrating molecular analysis into autopsies, we can significantly enhance diagnostic capabilities, provide vital information to affected family members, and advance our understanding of genetic diseases. This paradigm shift towards utilizing postmortem NGS holds immense potential for revolutionizing forensic medicine and contributing to a comprehensive investigation of SCD.




