Fatal Pneumonia Caused by Omicron Subvariant BA.2 of Severe Acute Respiratory Syndrome Coronavirus 2 with the Pulmonary Tuberculosis
Article information
Trans Abstract
The coronavirus disease 2019 (COVID-19) pandemic resulted from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Variants of SARS-CoV-2 have caused distinct COVID-19 surges worldwide. The Omicron variant has replaced other variants as a cause of COVID-19 in the Republic of Korea. Fortunately, COVID-19 patients infected with Omicron have a decreased disease severity. Tuberculosis (TB) remains a major public health threat worldwide, and the incidence of TB is still high in the Republic of Korea. We report the case of a deceased illegal migrant who died at home. An autopsy revealed fatal pneumonia with pulmonary TB caused by the Omicron subvariant BA.2 of SARS-CoV-2. We assumed that a superimposed SARS-CoV-2 infection caused this fatal pneumonia with a previous TB infection. After a comprehensive postmortem (PM) examination, including gross dissection, microscopic studies, PM computed tomography, and PM laboratory tests, the cause of death was determined to be pneumonia, and the death manner was natural. We present this case with a comprehensive PM examination from the perspective of forensic pathology and the public healthcare system.
Introduction
The coronavirus disease 2019 (COVID-19) pandemic resulted from the spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Variants of SARS-CoV-2 have caused distinct COVID-19 surges in the world. Following Alpha and Delta, the Omicron variant replaced the other variants as the primary cause of COVID-19 in the Republic of Korea. The combination of social distancing and widespread vaccination against SARS-CoV-2 has maintained a low rate of COVID-19 in the Republic of Korea. However, the Omicron variant has significantly increased vaccine breakthrough infection rates. Consequently, COVID-19 incidence has increased explosively owing to the Omicron variant of SARS-CoV-2 in the Republic of Korea. Fortunately, compared with patients infected with either Alpha or Delta variants, COVID-19 patients infected with Omicron were younger and significantly less likely to be hospitalized. In addition, patients infected with Omicron required less intensive respiratory support and had a shorter length of hospital stay, consistent with an average decrease in disease severity [1]. Nevertheless, it cannot be denied that COVID-19 can be fatal. The Omicron variant of SARS-CoV-2 is divided into subvariants, including BA.1 and BA.2. Of these, BA.2 is called the stealth Omicron because of the difficulty in its detection.
Despite great efforts, tuberculosis (TB) remains a major public health threat worldwide; the incidence rate of TB is still high in the Republic of Korea [2]. Herein, we present an autopsy case of fatal pneumonia caused by the Omicron subvariant BA.2 of SARS-CoV-2 with pulmonary TB. This case is presented from the forensic pathology perspective and could interest forensic pathologists. Moreover, this case report highlights points that should not be overlooked from the public healthcare system perspective.
Case Report
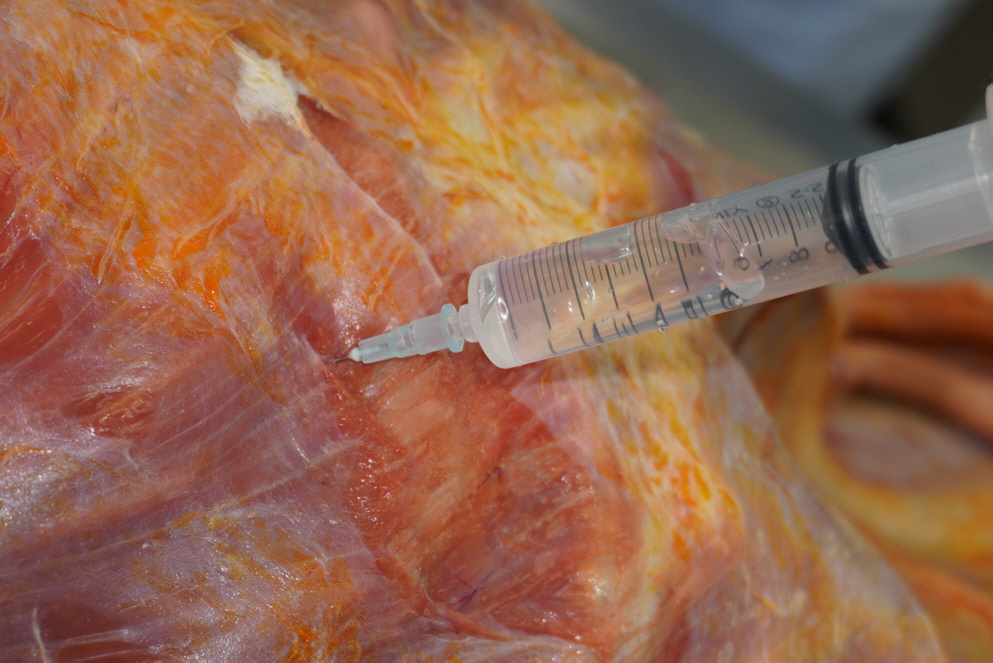
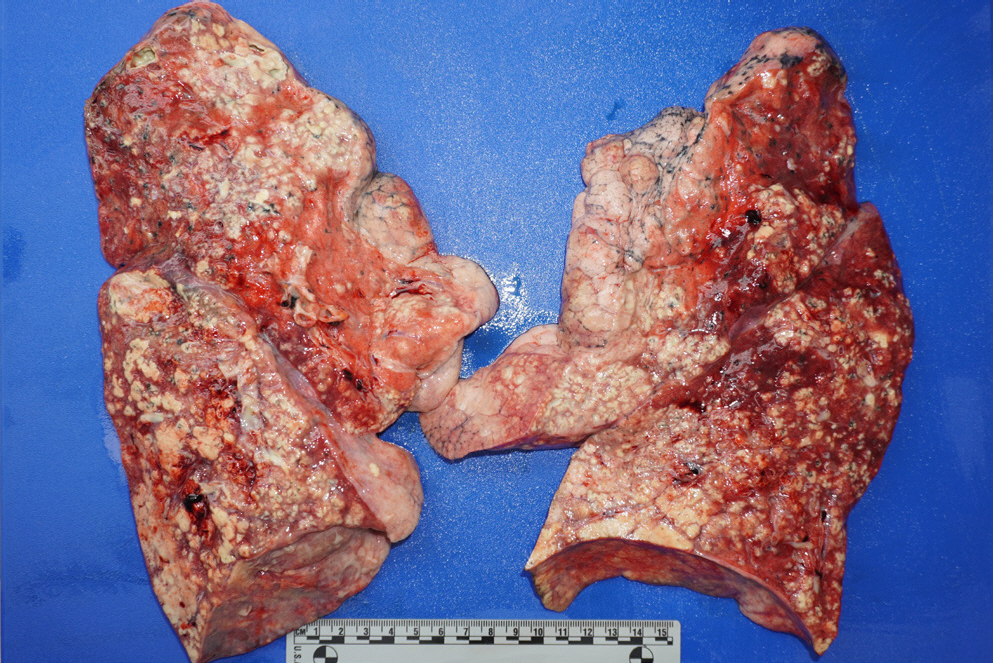
The patient was a 53-year-old man, an illegal migrant who died at home. A neighbor reported that he seemed sick and called for emergency services several times over 2 weeks. Unfortunately, the patient did not receive proper medical care for several reasons, including his illegal status as a migrant. The neighbor eventually asked the community service center for help, but he was dead when he was found. An autopsy was performed within 3 days after death with a court-issued warrant from the public prosecutor. The patient had a slim physique and a body mass index of 11.3. Several linear abrasions that appeared to be self-inflicted wounds were identified in the right wrist. External examination revealed no injuries other than the cardiopulmonary resuscitation marks. On internal examination, positive pneumothorax test results were noted on both sides of the chest (Fig. 1). A small amount of pleural effusion (approximately 100 mL each) was identified in both the thoracic cavity and the clotted blood in the cardiac lumen. The weights of the right and left lungs were 1,014 g and 680 g, respectively. Purulent sputum was noted in both the main bronchi and numerous abscess foci were identified in all lobes (Fig. 2). Examination of other internal organs was unremarkable. After gross dissection, the cause and manner of death were assumed to be pneumonia and natural conditions, respectively.
Postmortem computed tomography (PMCT) was performed before the autopsy. PMCT showed that the trachea was located in the midline, and pneumothorax was present on both sides of the thoracic cavity. In addition, livor mortis was observed in the dependent portions of both the lungs. Furthermore, the PMCT scan demonstrated chronic destructive TB with active disease due to non-specific causes (Fig. 3). Microscopic examination of the lungs showed multiple caseating necrotic tissues with granulomas, consistent with TB and widespread pneumonia in all lobes of both lungs. Moreover, the lung tissue showed diffuse alveolar damage (hyaline membranes, intra-alveolar and/or interstitial edema, proteinaceous, intra-alveolar fibrinous exudate, intra-alveolar mononuclear cell infiltrates, and type 2 pneumocyte hyperplasia/activation). In addition, fibrin-enriched microthrombi were identified in the lumen of some microvasculatures (Fig. 4).
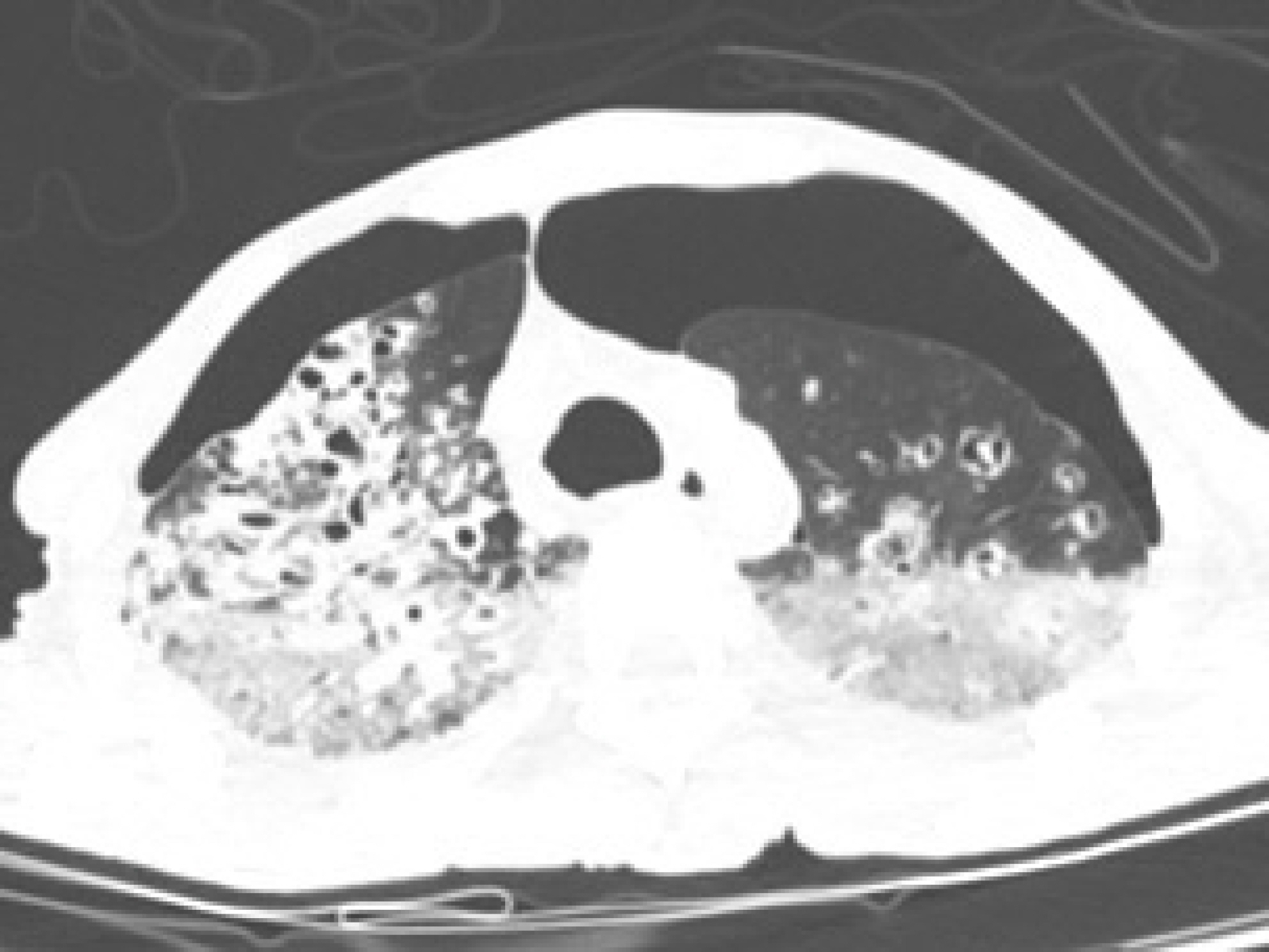
Postmortem computed tomography (PMCT) showed pneumothorax on both sides of the thoracic cavity. The trachea was located on the midline of the cervical and thoracic area. PMCT scan demonstrates bronchiectasis with cavitary destruction in both lungs, suggesting chronic destructive tuberculosis. In addition, tree-in-bud and centrilobular nodules observed in both lungs were significantly associated with active disease by a non-specific cause.
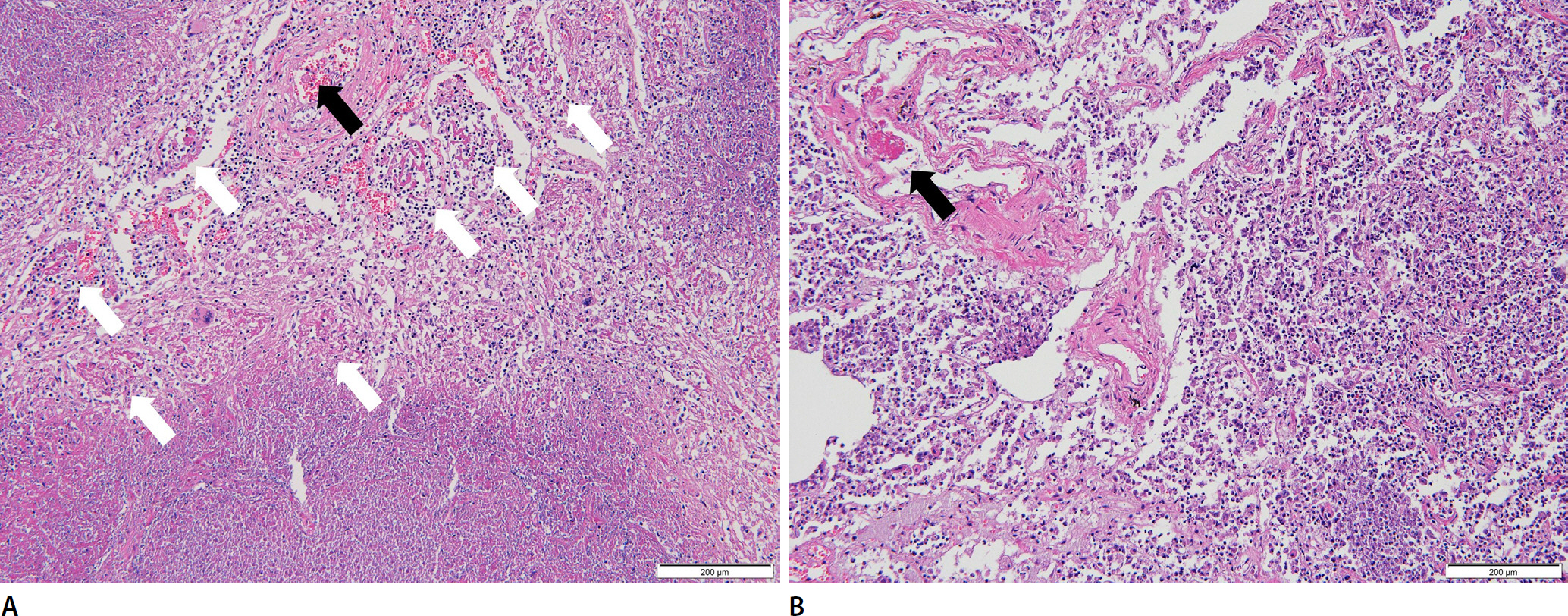
Granulomatous inflammation with caseating necrosis is noted. Diffuse alveolar damage with hyaline membranes, intra-alveolar and interstitial lymphocytic infiltration, and proteinaceous edema are also noted. Fibrin-enriched microthrombi are noted in the lumen of the microvasculature. Purulent pneumonia is also noted (A and B, H&E, ×100). White and black arrows indicate intra-alveolar hyaline membranes and microthrombi, respectively.
SARS-CoV-2 Omicron subvariant BA.2 was identified in the main bronchus's postmortem (PM) swab sample. However, SARS-CoV-2 was not detected in the pleural fluid of the PM. No major respiratory viruses were detected in the PM swab samples of the main bronchus by polymerase chain reaction, including adenovirus, enterovirus, influenza virus, parainfluenza virus, respiratory syncytial virus, and rhinovirus. Moreover, the bacterial culture test was negative for the PM swab samples from the main bronchus. Biochemical analysis of the vitreous humor revealed no remarkable findings. The blood C-reactive protein level was 5.73 mg/dL (reference range, less than 1.0 mg/dL), and the blood procalcitonin level was 0.34 ng/mL (reference range, less than 0.5 ng/mL). Toxicological tests using PM blood showed no significant findings (any drug or blood alcohol concentration was less than 0.010%). TB polymerase chain reaction using formalin-fixed paraffin blocks of the lungs was positive for Mycobacterium tuberculosis.
After comprehensive PM examination, including gross dissection, microscopic examination, PMCT, and PM laboratory tests, we concluded that the deceased died of fatal pneumonia caused by the Omicron subvariant BA.2 of SARS-CoV-2 with pulmonary TB. Finally, the manner of death was determined to be natural.
Discussion
Following Alpha and Delta, the Omicron variant became the primary cause of virtually all COVID-19 cases in the Republic of Korea. The first sequenced Omicron variant was reported in Botswana on November 11, 2021, and a few days later, another case was reported of a traveler from South Africa to Hong Kong [3]. The early doubling time in the wave by the Omicron variant was higher than that of previous waves [4]. The Omicron variant of SARS-CoV-2 is divided into subvariants including BA.1, BA.2, and other subvariants (XE, XL, XM, etc.) were also identified in the Republic of Korea. Of these, the BA.2 Omicron subvariant does not have the complete set of polymorphisms characteristic of BA.1 and has additional unique mutations. This subvariant lacks the spike gene deletion in the regions encoding amino acids 69 and 70. Hence, some commonly used assays will not detect it, such as the S-gene target-failure assay (TaqPath COVID-19 Combo kit, Thermo Fisher, Inc., Waltham, MA, USA) [1]. Difficulty in detection has earned it the name stealth Omicron. Omicron variants have some deletions and more than 30 mutations, several of which overlap with those in Alpha, Beta, Gamma, and Delta variants [3]. These deletions and mutations lead to increased transmissibility, higher viral binding affinity, and higher antibody escape [5,6]. Omicron variants spread faster and might escape antibodies more readily than previous variants, thereby increasing the cases of reinfection and mild breakthrough infections in vaccinated people [7]. Fortunately, most COVID-19 vaccines have remained effective and may depend more on T-cell immune responses than antibodies [7]. COVID-19, caused by the Omicron variant of SARS-CoV-2, is thought have a decreased disease severity [1]. Compared with patients infected with either Alpha or Delta variants, significantly fewer patients infected with Omicron were hospitalized, and those who were hospitalized required significantly less intense respiratory support and had a shorter length of admission. Many factors have contributed to this, including, but not limited to, increased vaccination uptake, population immunity, and patient demographics, such as younger age [1]. Nevertheless, it cannot be denied that COVID-19 can be fatal. A previous report showed that sustained pathophysiological changes in COVID-19 may increase the risk of sudden death due to intoxication [8]. The authors suggested that the potentially hazardous effects of persistent COVID-19 pathologies should be considered a contributing factor in individuals with competing explanations for the cause of death [8]. Furthermore, they suggested that PMCT is a powerful noninvasive screening tool that enables safe practices in potentially infectious cases and allows forensic pathologists to alter dissection approaches as appropriate [8]. Before beginning the dissection, we were aware from the PMCT of the pneumothorax and pneumonic infiltration in the present case. Therefore, the autopsy was performed with caution.
Macroscopic examinations of lungs infected with SARS-CoV-2 often show heavy, edematous, and congested lungs [9]. The present-case deceased had a low body mass index; however, both sides of the lung showed macroscopic findings similar to those of previous reports, and the weights of the right and left lung were 1,014 g and 680 g, respectively. The most frequently encountered histological finding in SARS-CoV-2 infected lung is diffuse alveolar damage at different stages, mainly in exudative and proliferative phases, characterized by hyaline membranes, intra-alveolar and/or interstitial edema, proteinaceus, intra-alveolar fibrinous exudate, intra-alveolar mononuclear cell infiltrates (macrophages, lymphocytes, and neutrophils), type 2 pneumocyte hyperplasia/activation, and squamous metaplasia [9]. In several cases, fibrin-enriched thrombi in vessels have been reported, mostly appearing as microthrombi in alveolar capillaries and/or small vessels [9]. Previous studies have reported similar findings based on PM examination [10,11]. These findings were also noted in the present case. Additionally, some reports have described focal or diffuse pneumonia or bronchopneumonia [9]. A case of severe pneumonia due to the Omicron variant of SARS-CoV-2 has also been reported [12]. In this case, numerous abscess foci were identified in all lobes, and microscopic examinations revealed multiple caseating necrotic tissues with granulomas, consistent with TB and widespread pneumonia in all lobes of both lungs. The authors assumed that these pathological findings were caused by superimposed SARS-CoV-2 infection in the destructive pulmonary pathology caused by chronic TB. Furthermore, a previous report suggested that persistent sequelae of COVID-19 may pose a sustained risk factor for sudden death in individuals recovering from the acute phase of the disease [8].
Sudden death due to pulmonary TB is rare in forensic practice in the Republic of Korea, although there is a high incidence of TB infection. COVID-19, caused by the Omicron variant of SARS-CoV-2, has been regarded as less severe than COVID-19 caused by other variants. However, TB is still a common infectious microorganism, and this report shows fatal pneumonia of the COVID-19 caused by the Omicron subvariant BA.2 of SARS-CoV-2 with pulmonary TB. This report highlights some significant points for forensic medicine and public healthcare systems. First, it should be noted that COVID-19 caused by the Omicron variant can be fatal when accompanied by other underlying diseases, such as TB. Second, infectious diseases do not distinguish between domestic and foreign individuals. Domestic people can spread the disease to foreigners, and the reverse is equally possible. Therefore, a public healthcare system should include all people. Third, autopsy staff should be protected from potential infectious agents during an autopsy, particularly during pandemics. Studies identifying PM samples with potential contagion should be continuously conducted, and autopsy staff should be alerted to potential contagious PM samples. In this case, SARS-CoV-2 was detected in the bronchial swab sample but not in the PM pleural fluid samples obtained during the autopsy. Like the present case, COVID-19 is strictly related to a hyper-inflammatory state that starts with diffuse alveolar damage and immunothrombotic microangiopathy [8]. Finally, we highlight the importance of autopsy to provide a fundamental basis for understanding the consequences of SARS-CoV-2 infection, referring to previous reports [8,9].
Notes
Conflicts of Interest
Joo-Young Na, a contributing editor of the Korean Journal of Legal Medicine, was not involved in the editorial evaluation or decision to publish this article. All remaining authors declare that there is no conflict of interest.
Acknowledgments
This study was supported by a 2022 research grant from Pusan National University Yangsan Hospital.